
Lea esta hoja informativa en español
Table of Contents
- Different Kinds of Cure
- Latency Reversing Agents
- Block and Lock Strategies
- Gene Therapy
- Immune-Based Approaches
- Combination Approaches
Although a few people appear to have been cured of HIV under special circumstances, there is currently no available cure for HIV. See our fact sheet on Finding a Cure for HIV for information on general challenges to developing such a cure and what the research means for women. The current fact sheet provides information on what scientists are trying to do to develop a cure.
Different Kinds of Cure
There are several terms currently used in HIV cure-related research. All of them assume that a person no longer needs to take today's HIV drugs, at least for long periods of time:
- Elimination or virologic cure – getting rid of all virus from all locations in the body
- Durable antiretroviral (ART)-free control – HIV may still be in the body, but it is not active; the body is not fully rid of HIV, but the virus cannot affect one's health and cannot be transmitted to others; also sometimes called "post-intervention control"
Read on for the different strategies that could be used to achieve one of these types of cure. All are still being researched; some are at more advanced research stages than others. Each strategy includes different ways to achieve the same thing – e.g., for "latency reversal agents," different substances could be used to "awaken" the hidden virus. There are also challenges with each of these strategies, which are explained below.
Latency Reversing Agents
Also sometimes called "poke and clear," the game plan here is to "poke" the resting cells in the reservoirs into action, then "clear" the newly activated cells when HIV returns to the bloodstream. Once the cells become active, they are no longer hidden from the immune system. The substances that re-awaken the resting HIV are called latency reversing agents, as they interrupt HIV's ability to remain inactive within cells.
At the same time, regular antiretroviral therapy would prevent cells that do not have HIV from newly acquiring HIV. Ideally, this strategy would empty the reservoirs and thereby rid the body of HIV. Latency reversing agents would need to be taken along with strategies that enhance the immune system.
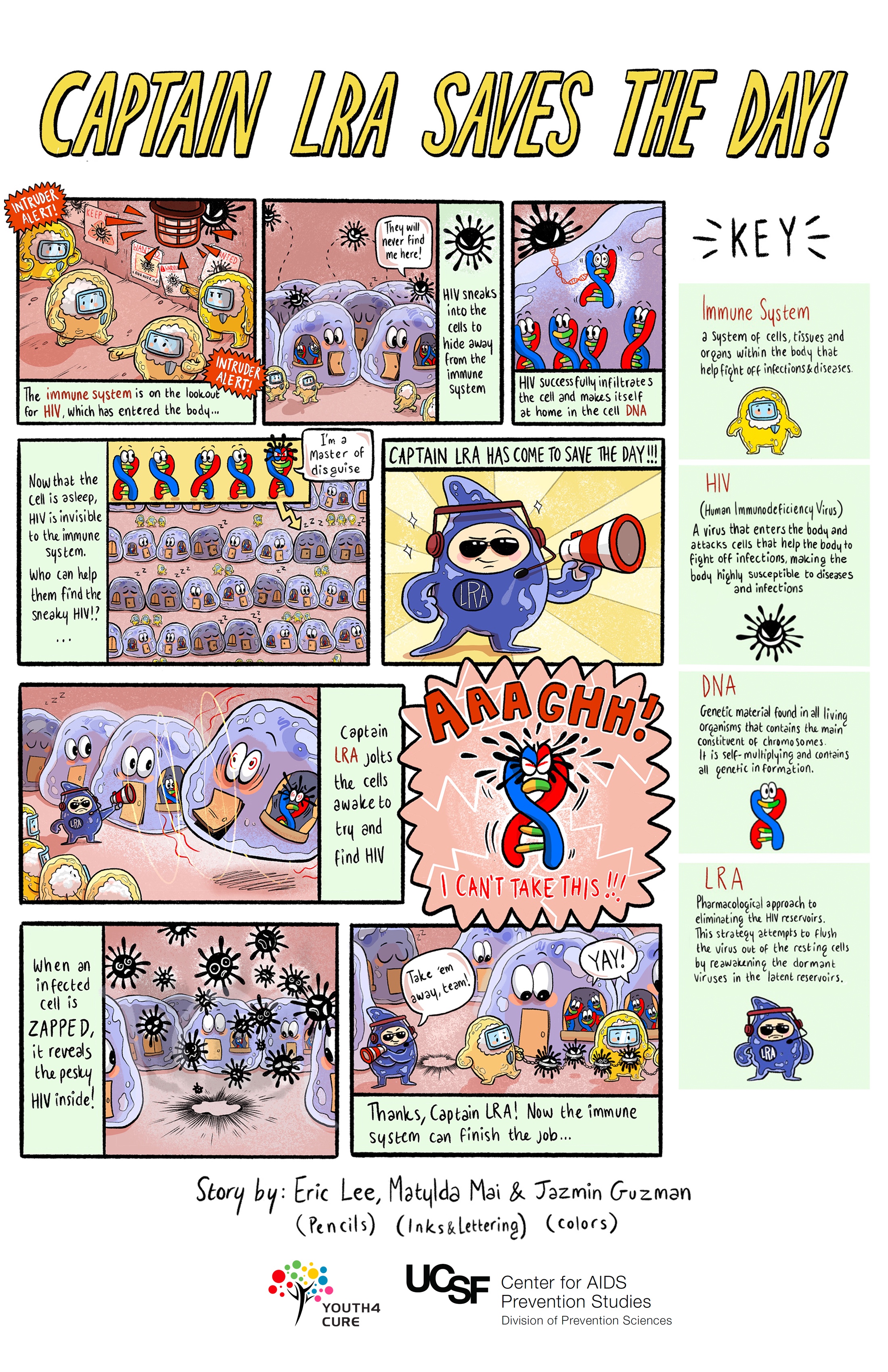
Challenges with this approach:
- Finding substances (latency reversing agents) that will safely and effectively activate, or reawaken the resting cells into action
- Ensuring that every cell with HIV is reactivated when poked, leaving no harboring cell untouched
- Ensuring that cells with HIV that are re-awakened to start producing virus end up being cleared from the body; approaches to bolstering the immune system's ability to recognize and clear the newly activated cells are listed below.
Block and Lock Strategies
Rather than activating and clearing off cells as with latency reversal strategies, the "block and lock" approach aims to permanently silence latent (inactive) virus using latency promoting agents (LPAs) to "block" the transcription step in the cell's lifecycle. Over time, this would "lock" the part of the cell or the gene responsible for HIV transcription into a deep latent state. Permanent control of that part of the cell means HIV drugs would no longer be necessary. Another key aspect of these "block and lock" approaches is that the agent would be specific to HIV (e.g., Tat gene), so it would not block other processes needed to overcome other infections.
Studies in humans will be needed to test whether this kind of approach could work to achieve durable antiretroviral treatment-free control. However, unlike latency reversal approaches that are studied in HIV after being used to manage other health conditions, no block and lock strategy has been approved by the US Food and Drug Administration (FDA). Therefore, clinical trials of these therapies have lagged behind.
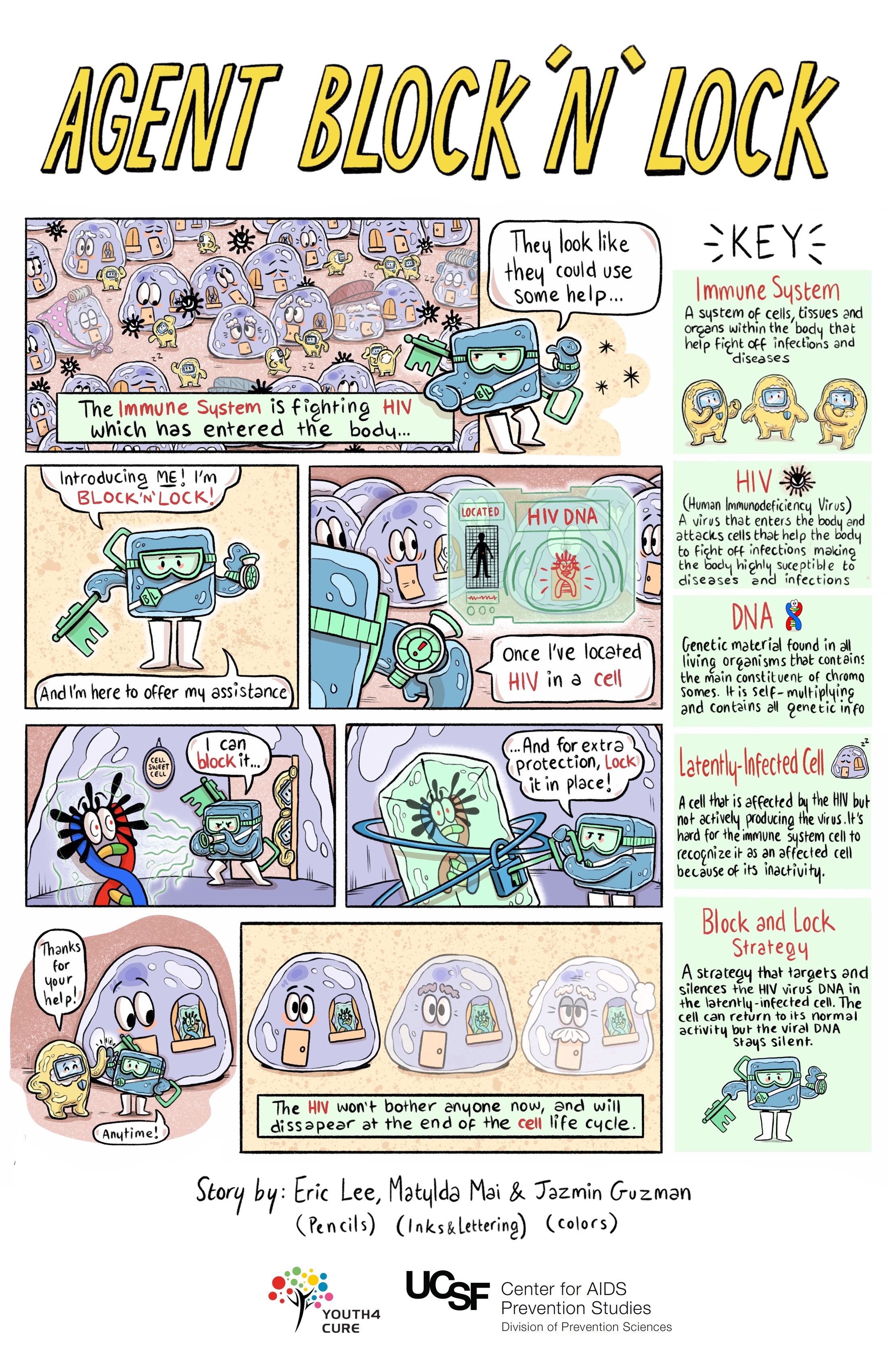
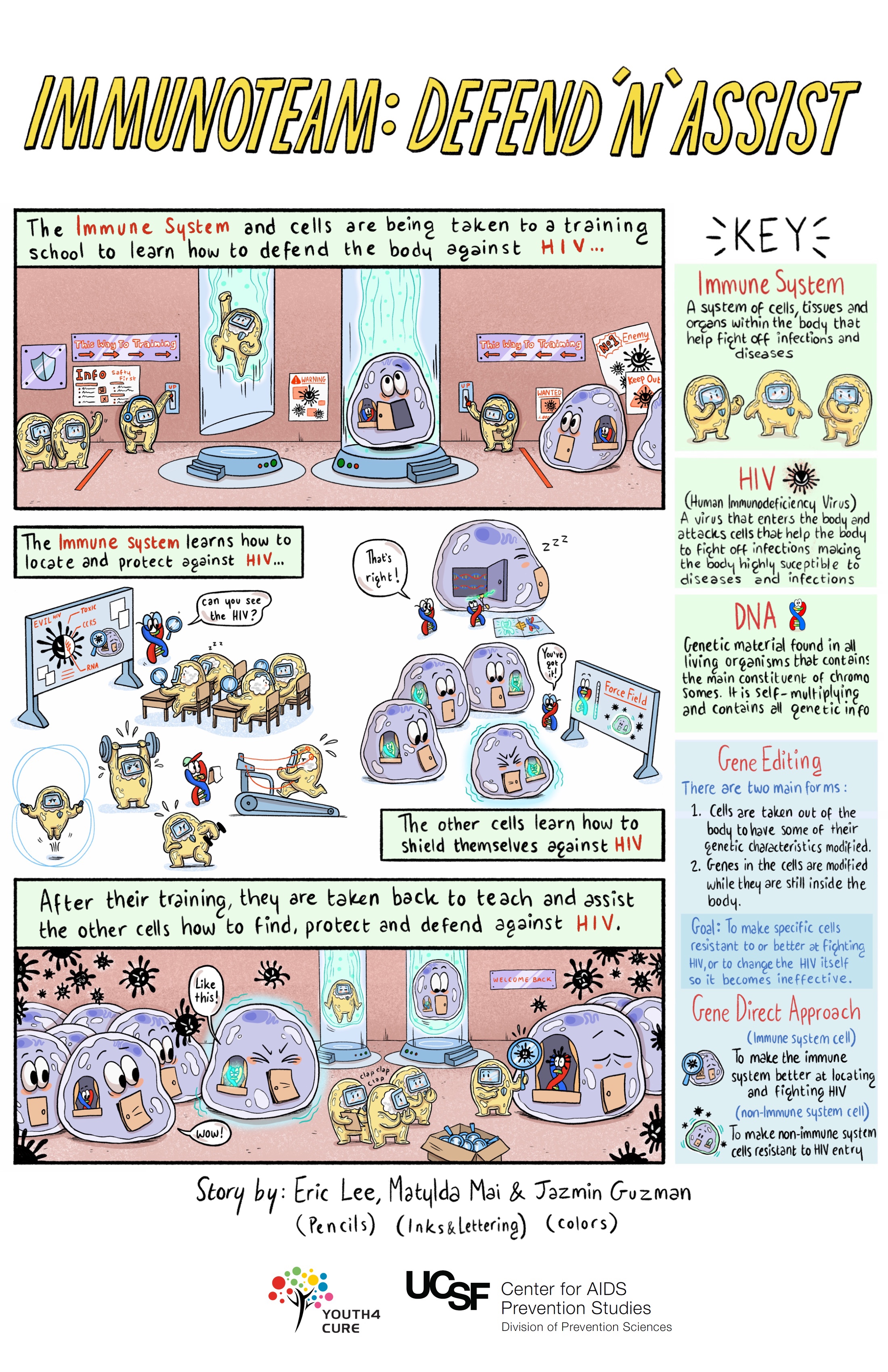
Gene Therapy
There are three broad approaches to gene therapy:
- knocking out genes in the virus that allow it to enter immune cells;
- knocking in genes to our immune cells that make them resistant to HIV; and
- cutting out the genetic pieces of HIV that have become integrated into the DNA of harboring immune cells.
Knocking Out Genes in the Virus
The first strategy looks to disable HIV and make it unable to enter cells in your body by editing the genetic code of the virus. The genes that code for or provide the manufacturing instructions for HIV's ability to enter cells would be deleted or "knocked out." Thus, HIV would remain in the body, but it would be unable to enter cells in your or other people's bodies.
Adding Genes to Immune Cells
The second approach involves adding, or "knocking in" genes to a person's immune cells that would protect them against HIV. We know what those protective genes look like because some people are naturally born with them. These people are protected by their inability to produce a receptor called CCR5 on the outside of their immune cells that HIV needs to enter the cells.
One popular example of this approach is Timothy Brown, once known as the "Berlin patient." He received a stem cell transplant after he was diagnosed with leukemia (a form of blood cancer). Stem cells are cells that have not yet received instructions from the body to make them into a specific type of cell. Stem cells can renew themselves, develop and grow into several different types of cells, including several different kinds of immune cells.
The stem cells Brown received were from a person who was naturally protected from HIV by having genes that lacked the ability to produce a functional CCR5 receptor, one of two T-cell receptors that HIV needs to bind to in order to enter cells. Brown was considered cured of HIV, though he died of cancer in September 2020.
A similar procedure appears to have been successful in four other people:
- The first one is known as the "London patient" – a man named Adam Castillejo
- The second person, previously called the "Düsseldorf patient" and now known to be a man named Marc Franke, also received a bone marrow transplant to treat leukemia. He was declared cured of HIV in 2023.
- In early 2022, researchers announced the first woman to reach durable ART-free control of HIV following a procedure. The "New York patient," who at this point wishes to remain anonymous, is of mixed race and was also diagnosed with leukemia. She underwent a transplant using stem cells from umbilical cord blood, but from an individual with a CCR5 mutation like in the three previous cases. She remains in post-intervention control without HIV medications at the time of this writing.
- A second woman, known as the "French patient," received a stem cell transplant to treat leukemia in July 2020, after ten years with an undetectable viral load. The donor cells also had the CCR5 mutation. Within months, researchers could not find HIV in her CD4 cells or blood plasma. She chose to stop taking ART in October 2023 and remains HIV-free as of this writing, with continued monitoring to eventually determine whether she can be declared "cured."
In mid-2022, a potential cure of an older person – Paul Edmonds, once known as the "City of Hope patient" – was announced. He had lived with HIV for a long time, and after a stem cell transplant has been free of HIV without treatment since 2021.
Two other people living with HIV and cancer who received stem cell transplants to treat their cancer are commonly referred to as the "Boston patients." These people stopped taking HIV drugs for a couple of years after their transplants and were able to go for several weeks or months without the virus reappearing. Unlike Timothy Brown, however, they did not receive donor stem cells from a person resistant to HIV. As a result, although they appeared to have no evidence of HIV after their transplants, the virus ultimately returned in both of them after they stopped taking their HIV drugs.
Another person with cancer who had a stem cell transplant, the "Geneva patient," has remained without the virus after stopping ART. He had started HIV treatment early and had an undetectable viral load for a long time before receiving a transplant from a donor who did not have the HIV-protective CCR5 mutation. His viral load remains undetectable, but very small amounts of defective virus remain. Researchers therefore call this a case of HIV remission, not cure. A similar approach was used with another person, the "second Berlin patient" whose HIV also remains in post-intervention control.
Thus far in 2025, two new cases have been reported:
- The "Chicago patient" is a 67-year-old man who was treated for leukemia with a stem cell transplant with donor cells that were resistant to HIV. More than a year after his transplant, no HIV could be found in circulating cells in his blood or bone marrow. When his ART was stopped, however, HIV from his reservoir returned. The researchers restarted ART, but stopped again after two years, and this time the man achieved HIV remission for the time being. This marks the first known case of sustained HIV remission without HIV treatment after post-transplant viral rebound.
- The "Oslo patient," who had been living with HIV for 14 years, was diagnosed with a blood disorder other than leukemia, called myelodysplastic syndrome. While he does not have HIV-resistant cells, his brother was found to have them, and donated stem cells for his transplant. The man stopped treatment two years after the procedure and has been in remission for four years. This marks another “first”: the first case of HIV remission using donor cells from a sibling.
Cutting Out Genetic Pieces of HIV
The third gene therapy approach uses relatively new technologies (e.g., CRISPR) that are able to latch precisely onto the HIV genes that have integrated into human DNA and cut them out without harming the cell or causing it to malfunction.
Challenges with these approaches:
- Changing the genetic sequence or code can produce unexpected results, including unintended side effects
- Stem cell therapies require wiping out a person's existing immune cells. This process can involve several types of drugs as well as radiation to create a 'clean slate' for the newly transplanted stem cells to flourish and grow. It is a lengthy, uncomfortable, and dangerous process. In addition, there are few individuals naturally immune to HIV (few potential stem cell donors), and the process is very costly.
- Virus-removal techniques would need to be highly specific (removing only HIV genetic material and not human genetic material) and highly sensitive (able to find almost all the cells with HIV)
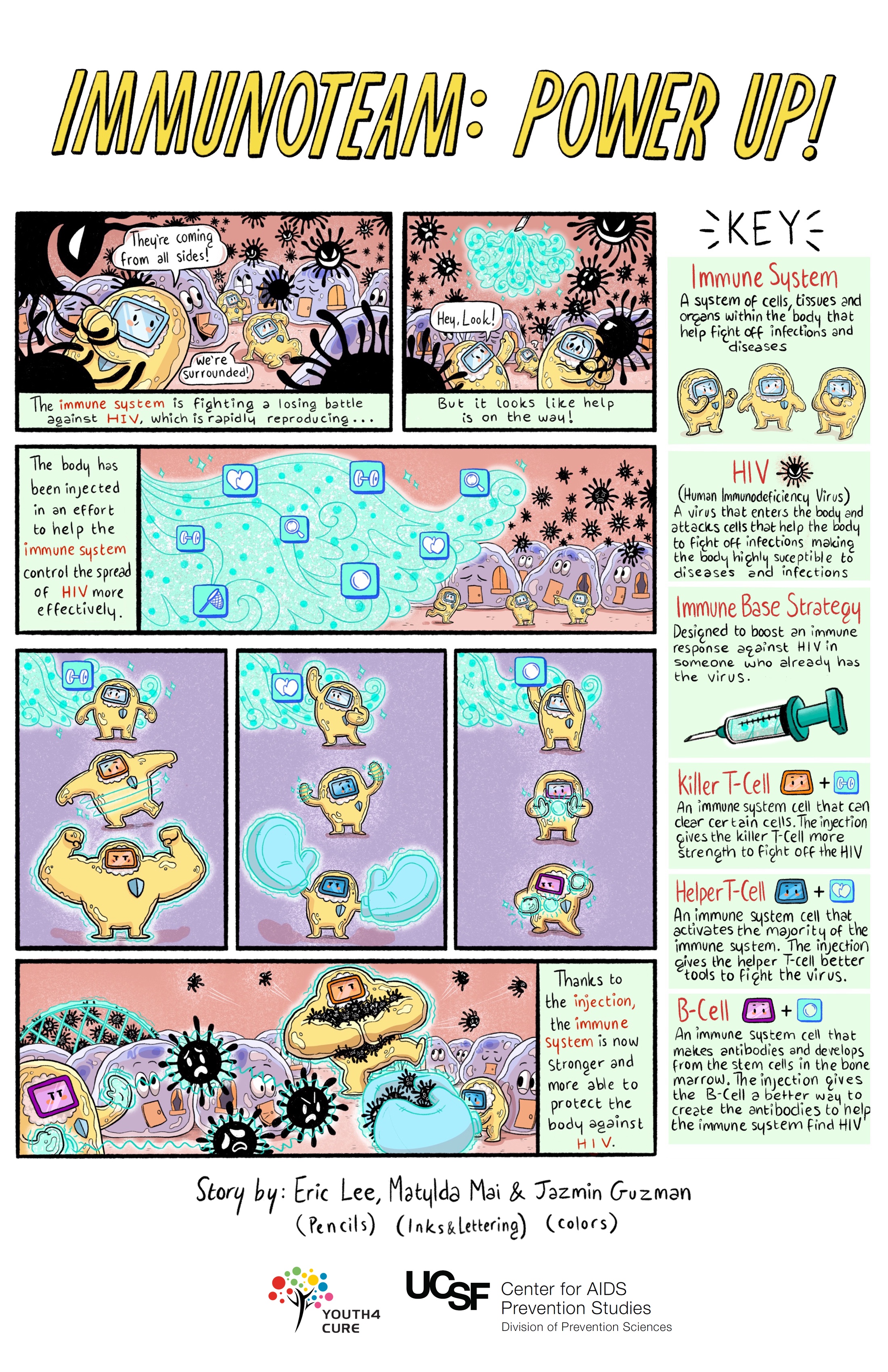
Immune-Based Approaches
Immune-based approaches work by enhancing the immune system's ability to clear cells harboring HIV and achieve a cure. Scientists are looking at three main approaches:
- Broadly neutralizing antibodies (bNAbs): When our immune cells clear pathogens like HIV, they display pieces of the virus — known as antigens (from "antibody-generating") — on their surfaces. An antibody is a protein that attaches to an antigen like a key fits a lock. When an antibody has matched up with an antigen, it has marked the intruder for destruction by immune cells. Broadly neutralizing HIV antibodies can recognize and target for destruction several different strains of HIV, unlike standard antibodies, which are usually only able to latch onto antigens from a single strain of the virus.
- Natural killer (NK) cells and interferon-gamma: NK cells destroy cells with HIV and are an important part of the body's early response to viral infections. They clear cells while the body is prodding "killer" (cytotoxic) T cells into action. Unlike some immune cells, NK cells do not need to have HIV to effectively identify or clear HIV. Scientists are hoping that this new understanding of how NK cells work can lead to a therapeutic vaccine or durable ART-free control of HIV.
- Chimeric antigen receptor (CAR) T-cell therapy: In this approach, the T cells of a person living with HIV would be engineered to have receptors that reprogram those cells to identify and eliminate cells with HIV. The FDA has approved this therapy in a certain type of leukemia, and it is being studied in other kinds of cancers. There have been promising studies in animal models. Clinical trials exploring CAR T cells as an approach to curing HIV have begun in humans.
About 0.5 percent of people living with HIV around the world who acquired HIV continue to have low viral loads and high CD4 counts without ever taking HIV drugs. They are called "elite controllers." One of these is Loreen Willenberg – also known as the "San Francisco patient." After acquiring HIV in 1992, but not progressing to worse HIV disease or AIDS, she participated in research studies for many years, hoping that a path to an HIV cure might include what scientists could learn from her unique cells. In 2011, researchers realized they could not find any virus inside Willenberg's body that could make copies of (replicate) itself – including in reservoirs. The virus had entirely cleared from her body by itself.
In 2021, this was found to have occurred with a second woman in Argentina, known as the "Esperanza patient." Now, the job of scientists is to figure out how to repeat the process in other people. These cases are inspiring research efforts towards "block and lock" approaches (see above) to keep HIV from replicating inside the body.
Challenges with immune-based approaches:
- Both broadly neutralizing antibodies and "post-treatment controller" NK cells are hard to find. They occur in only a small minority of people and may not be able to get into all parts of the body where HIV hides.
- Stimulating the immune system can increase the number of cells that HIV can target
- There are many different strains of HIV, and HIV mutates, or changes, very rapidly. This may make even broadly neutralizing antibodies ineffective over time. Experts believe people may need to receive a combination of broadly neutralizing antibodies just as today's effective HIV treatment regimens include multiple drugs.
Combination Approaches
Most likely, we will need a combination of approaches to achieve ongoing control of HIV without HIV drugs. Scientists are investigating safe ways to combine different HIV cure research approaches.
The Well Project would like to thank Karine Dubé, DrPH (University of California San Diego and UNC-Chapel Hill), for thorough review of updates to the fact sheet content in 2025 and 2022; Danielle M. Campbell, MPH (Charles R. Drew University), for her significant contribution to the 2022 fact sheet review; as well as Rowena Johnston, PhD (amfAR) and David Evans (Project Inform) for their assistance authoring the original edition of this fact sheet.

